Sun, Dec 29, 2024
[Archive]
Volume 14, Issue 4 (December 2017)
IJMSE 2017, 14(4): 35-47 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shahraki M, Habibi-Khorassani S M, Noroozifar M, Yavari Z, Darijani M, Dehdab M. CORROSION INHIBITION OF COPPER IN ACID MEDIUM BY DRUGS: EXPERIMENTAL AND THEORETICAL APPROACHES. IJMSE 2017; 14 (4) :35-47
URL: http://ijmse.iust.ac.ir/article-1-876-en.html
URL: http://ijmse.iust.ac.ir/article-1-876-en.html
Abstract: (20959 Views)
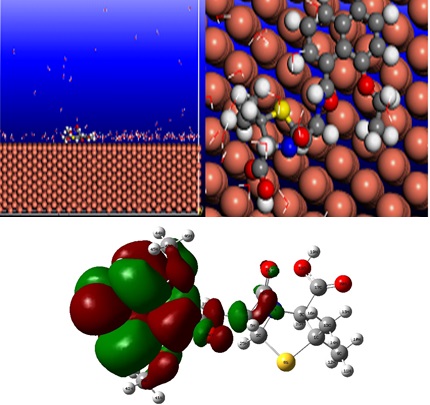
The inhibition performances of nafcillin (III), methicillin (II) and penicillin G (I) on the corrosion of copper in HCl was studied and tested by weight loss, Tafel polarization, SEM, UV-vis spectrophotometry, molecular dynamics method and quantum chemical calculations. Polarization curves indicated that the studied inhibitors act as mixed-type inhibitors. The values of inhibition efficiency and surface coverage were found to follow the order: Blank ads, indicated that the adsorption of three inhibitors was a spontaneous process. The SEM micrographs confirmed the protection of copper in a 1 M HCl solution by penicillin G, nafcillin, and methicillin. The shape of the UV/vis spectra of inhibitors in the presence of the immersion of Cu showed a strong support to the possibility of the chemisorbed layer formation on Cu surface by nafcillin (between nafcillin and Copper) and physisorption between penicillin and methicillin with copper. DFT calculations were performed to provide further insight into the inhibition efficiencies which were determined experimentally. Molecular dynamics (MD) simulations were applied to find the most stable configuration and adsorption energies of penicillin G, nafcillin and methicillin molecules on Cu (110) surface. The interaction energy followed the order: nafcillin (III)> methicillin (II)> penicillin G (I), which confirmed that nafcillin has the strongest interaction with the metal surface. The obtained results from experimental and theoretical methods were in reasonable agreement.
Type of Study: Research Paper |
Subject:
Surfe coating and corrosion
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |